
The Horse:Your Guide To Equine Health Care provides monthly equine health care information to horse owners, breeders, veterinarians, barn/farm managers, trainer/riding instructors, and others involved in the hands-on care of the horse.
Issue link: https://thehorse.epubxp.com/i/858568
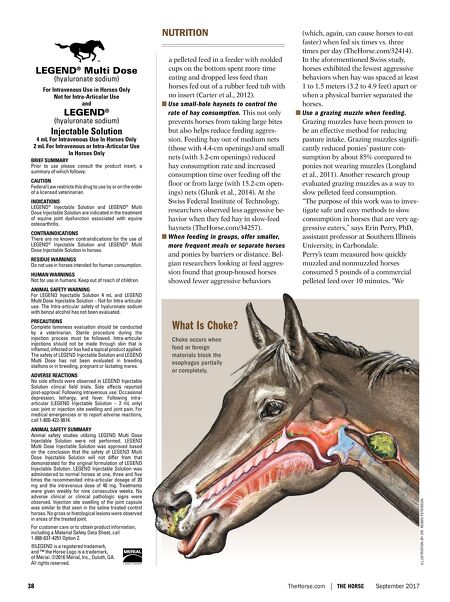
38 TheHorse.com THE HORSE September 2017 a pelleted feed in a feeder with molded cups on the bottom spent more time eating and dropped less feed than horses fed out of a rubber feed tub with no insert (Carter et al., 2012). ■ Use small-hole haynets to control the rate of hay consumption. This not only prevents horses from taking large bites but also helps reduce feeding aggres- sion. Feeding hay out of medium nets (those with 4.4-cm openings) and small nets (with 3.2-cm openings) reduced hay consumption rate and increased consumption time over feeding off the floor or from large (with 15.2-cm open- ings) nets (Glunk et al., 2014). At the Swiss Federal Institute of Technology, researchers observed less aggressive be- havior when they fed hay in slow-feed haynets (TheHorse.com/34257). ■ When feeding in groups, offer smaller, more frequent meals or separate horses and ponies by barriers or distance. Bel- gian researchers looking at feed aggres- sion found that group-housed horses showed fewer aggressive behaviors (which, again, can cause horses to eat faster) when fed six times vs. three times per day (TheHorse.com/32414). In the aforementioned Swiss study, horses exhibited the fewest aggressive behaviors when hay was spaced at least 1 to 1.5 meters (3.2 to 4.9 feet) apart or when a physical barrier separated the horses. ■ Use a grazing muzzle when feeding. Grazing muzzles have been proven to be an effective method for reducing pasture intake. Grazing muzzles signifi- cantly reduced ponies' pasture con- sumption by about 85% compared to ponies not wearing muzzles (Longland et al., 2011). Another research group evaluated grazing muzzles as a way to slow pelleted feed consumption. "The purpose of this work was to inves- tigate safe and easy methods to slow consumption in horses that are very ag- gressive eaters," says Erin Perry, PhD, assistant professor at Southern Illinois University, in Carbondale. Perry's team measured how quickly muzzled and nonmuzzled horses consumed 5 pounds of a commercial pelleted feed over 10 minutes. "We NUTRITION ILLUSTRATION BY DR. ROBIN PETERSON LEGEND ® Multi Dose (hyaluronate sodium) For Intravenous Use in Horses Only Not for Intra-Articular Use and LEGEND ® (hyaluronate sodium) Injectable Solution 4 mL For Intravenous Use In Horses Only 2 mL For Intravenous or Intra-Articular Use In Horses Only BRIEF SUMMARY Prior to use please consult the product insert, a summary of which follows: CAUTION Federal Law restricts this drug to use by or on the order of a licensed veterinarian. INDICATIONS LEGEND ® Injectable Solution and LEGEND ® Multi Dose Injectable Solution are indicated in the treatment of equine joint dysfunction associated with equine osteoarthritis. CONTRAINDICATIONS There are no known contraindications for the use of LEGEND ® Injectable Solution and LEGEND ® Multi Dose Injectable Solution in horses. RESIDUE WARNINGS Do not use in horses intended for human consumption. HUMAN WARNINGS Not for use in humans. Keep out of reach of children. ANIMAL SAFETY WARNING For LEGEND Injectable Solution 4 mL and LEGEND Multi Dose Injectable Solution – Not for Intra-articular use. The Intra-articular safety of hyaluronate sodium with benzyl alcohol has not been evaluated. PRECAUTIONS Complete lameness evaluation should be conducted by a veterinarian. Sterile procedure during the injection process must be followed. Intra-articular injections should not be made through skin that is inflamed, infected or has had a topical product applied. The safety of LEGEND Injectable Solution and LEGEND Multi Dose has not been evaluated in breeding stallions or in breeding, pregnant or lactating mares. ADVERSE REACTIONS No side effects were observed in LEGEND Injectable Solution clinical field trials. Side effects reported post-approval: Following intravenous use: Occasional depression, lethargy, and fever. Following intra- articular (LEGEND Injectable Solution – 2 mL only) use: joint or injection site swelling and joint pain. For medical emergencies or to report adverse reactions, call 1-800-422-9874. ANIMAL SAFETY SUMMARY Animal safety studies utilizing LEGEND Multi Dose Injectable Solution were not performed. LEGEND Multi Dose Injectable Solution was approved based on the conclusion that the safety of LEGEND Multi Dose Injectable Solution will not differ from that demonstrated for the original formulation of LEGEND Injectable Solution. LEGEND Injectable Solution was administered to normal horses at one, three and five times the recommended intra-articular dosage of 20 mg and the intravenous dose of 40 mg. Treatments were given weekly for nine consecutive weeks. No adverse clinical or clinical pathologic signs were observed. Injection site swelling of the joint capsule was similar to that seen in the saline treated control horses. No gross or histological lesions were observed in areas of the treated joint. For customer care or to obtain product information, including a Material Safety Data Sheet, call 1-888-637-4251 Option 2. ®LEGEND is a registered trademark, and ™ the Horse Logo is a trademark, of Merial. ©2016 Merial, Inc., Duluth, GA. All rights reserved. Choke occurs when food or foreign materials block the esophagus partially or completely. What Is Choke?